Pushing the Limits: a 90% Crystal Malt Beer
Crystal malt. These two simple words seem to elicit a fair amount of emotion in many homebrewers. Even though these malts have their place - few British beer styles, for instance, would be possible without them - it seems that, for whatever reason, many brewers view them with derision, as little better than a necessary evil - as an ingredient that should be minimized wherever possible.
Crystal malts are produced through a unique process; barley is steeped and germinated, then heated in a closed system that does not allow moisture to escape. The malt is held at temperatures where the amylase enzymes are activated, which converts the starches of the malt to simpler sugars - you may recognize this as the exact same reactions that take place during your mash. After this process, the grain is then kilned; the high heat caramelizes some of the sugars in the grain, which is why crystal malts are often referred to as "caramel" malts. The higher the kilning temperature, the darker the crystal malt; differences in kilning temperatures result in different flavor profiles from the caramelization that range from simple sweetness, to caramel, to toffee, to burnt sugar, to raisin and stone fruit. Crystal malts also contribute color - which is often varying in levels of red - to a beer.
While most homebrewing literature allows that crystal malts can be used at up to twenty percent of the grist, I have found that it is far more common for homebrewers to try to limit these malts to the five to ten percent range (or less). If I had a dollar for every time I've read testimonies of brewers who claim that even tiny amounts of the lowest lovibond rated crystal malts make beers just cloyingly sweet and undrinkble... well, I would have a little spending money, at any rate.
As it just so happens, caramel is perhaps my favorite flavor in the world. While the cool kids are ordering hot fudge sundaes, I'm the guy getting caramel on mine. I love caramel candies of all sorts (and please - don't ruin my perfectly good caramel with salt), so it should come as no surprise that I'm also fond of caramel flavor in beer. So much so, in fact, that my brewing buddies widely consider crystal malts to be my "thing".
I have never been shy about pushing beyond the commonly accepted usage thresholds for crystal malts, and even beyond the guidelines suggested by maltsters. One of my house beers is a British brown ale that features about twenty-two percent crystal malt. I have been told many times that surely my beer is undrinkably sweet - cloying and sticky are terms I've heard associated with it. Yet, the beer in question finishes below 1.010, ensuring that it is instead fairly dry. It does indeed have strong caramel flavor, but the beer isn't actually sweet by any stretch of the imagination.
A year ago, one of my brewing buddies latched on to some unclear language for the 2015 BrewUnited Challenge and decided that he should be allowed to brew a 100 percent C60 beer. We clarified the language, but I told him to go ahead and brew it if he wanted to do so, that I wouldn't disqualify him due to his technical loophole. Well, he backed off, but he did brew a 50 percent Special B beer (Special B is a very dark crystal malt, if you were not aware) for fun, and as a joke on me. We all assumed that his beer would be undrinkable, but he reported that while it wasn't really to his taste, it did present as a passable porter if you squinted at it just so.
A few weeks ago, I was mulling over some ideas for some fun beer experiments to try, why my friend's 50 percent Special B beer popped into my head. If he could brew a drinkable beer with 50 percent crystal malt, what was the actual ceiling?
And so, the idea for a new series of posts was born. I'm going to use the super unimaginative tag "Pushing the Limits" for these posts, in which I'll take some aspect of brewing and push it beyond the reasonable, widely accepted limits. If all goes well, I should be able to do about one of these experimental brews per month.
Homework on the subject of crystal malt led to some differing opinions. On the one hand, there is the opinion that I shouldn't even have to actually mash the crystal malt, as enzyme conversion already happened during the process outlined above. Another very common opinion is that despite this, crystal malt cannot self convert, that I would need a normal portion of base grain to ensure conversion of those sugars. My own LHBS owner believes that the very lightest C malts can be mashed successfully without much base malt, but that the darker ones will have no diastatic power at all, and will require base grains for success. Clearly, somebody is wrong somewhere!
My original idea was to go with a grain bill that was "only" 75 percent crystal malt, with the balance made up of Maris Otter. When I floated this silly idea to my brewing buddies on the BrewUnited email list, they mocked me a bit, but also encouraged me to go for broke (Marshall Schott of Brulosophy fame called me all sorts of names for not leaping right to 100% crystal malt). I ended up settling on a compromise of 90 percent crystal malt (split between C60 and extra dark crystal) and ten percent base malt. If this brew turns out successfully, I will bump the crystal malt percentage up - very likely all the way to 100 percent for a future experiment. If it fails, I'll dial things back a bit until I find the point where I do get successful conversion.
According to the most commonly repeated brewing wisdom, this brew should end in failure; the Maris Otter won't have enough leftover diastatic power to convert the sugars from such a high amount of crystal malt. It is also widely believed that my beer is going to finish with an unacceptably high final gravity and some overwhelming sweetness. Some other experiments suggest that the conventional wisdom is wrong, that enzyme conversion really does happen during the production process, that all I need to do is extract those sugars. Experience also tells me that crystal malts may slightly raise final gravity, but that it should be possible to make a beer with a lot of crystal malt that avoid being cloyingly sweet. There is, of course, one way to find out!
Understand that while the muse had in fact grabbed my imagination, there was no way that I was going to risk a full five gallon batch on a silly idea like this. Instead, I decided to brew a tiny, simple batch - I would brew this as a full volume BIAB batch on my stovetop, do no sparge, then dump it into one of my little two gallon fermentation buckets. I'll keg it up when fermentation is complete, and I should end up with about sixteen glasses worth of beer when I'm done. I can pull a few glasses from the keg, and if it's worth keeping, I'll bottle the remainder out of the keg. That way, I can turn it over quickly for another experiment for next month's brewday. Scaling things down like this also made it possible for me to tack this brew onto my normal planned brewday; this month, I was brewing my British brown ale for NHC. This really fit perfectly well, as I was able to bump the size of my starter up a bit and have yeast ready for both batches.
Brewday came, and my wife had left to go eat and shop with her mother and sister. Since she's not a huge fan of brewing smells, I decided that it made more sense to do the experimental (indoor) batch first. I pulled up Bru'n Water, punched in my grain bill... and started to panic.
Even with zero acid additions, my predicted mash pH was a ridiculously low 4.4. I sent a frantic email to the guys on the BrewUnited email list, explaining my dilemma. How could I add alkalinity without chalk or pickling lime? I did have calcium carbonate, which I was sure added alkalinity, but there wasn't a spot on Bru'n water for it. Oh noes!
Matt Chrispen of Accidentalis was kind enough to point out to me (without insult, even) that calcium carbonate is chalk. Ahem. I knew that. At least the guys didn't mock me like I really deserved.
This did lead to an interesting conundrum. Adding enough chalk to raise the pH to an acceptable level meant that my calcium concentration was going to be higher than I had ever heard of. Check this water profile:
To achieve this water profile (the brown malty profile in Bru'n water was my target), I ended up needing nearly nine grams of salts for my paltry 1.75 gallon batch - of which, almost six grams were calcium carbonate alone. This seemed crazy to me.
I added campden to treat the water, then stirred in the salts. The results were... interesting.

That doesn't look super appetizing...
The grains were dumped into my Brew Bag. I was a little worried as my Brew Bag is sized for my big Coleman xTreme cooler, but there was no way I was using my "plain Jane" grain bag with its big, ugly mesh.
Mashing in was pretty simple. I did overshoot my initial strike temperature, but I sat the pot into a sinkful of cool tap water for a couple of minutes, and was soon exactly where I wanted to be.

Target of 148 F? For a "for fun" stovetop batch, I'll take it.
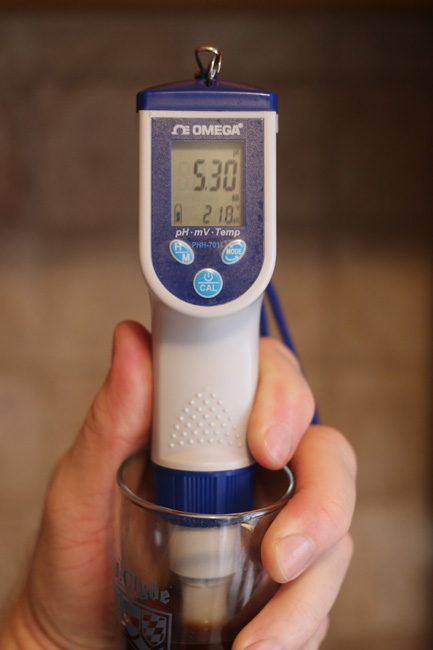
I then put the lid on the pot and wrapped it in towels. Despite all my chalk, my ten minute mash pH check came in low (thanks, water company!), but not so low that I was worried.
For a while, I started to think that this super simple method had a lot of appeal. Then, thirty-five minutes in, I checked my mash temp and found it lower than I wanted - 143 degrees F. So I removed the towels and turned the heat on. In virtually no time, I was at a freaking 160 F. I ran cool water and stuck the pot in, got it back to 148 F, and replaced the towels. Now, I remembered the biggest drawback to this method - the difficulty in maintaining mash temperatures!
When the mash was over, I removed the grain bag (making a bit of a sticky mess on the countertop). At least the Brew Bag did keep grain out of the boil, and was super easy to clean for the next batch of the day. I then added my hops (I did one FWH addition of East Kent Goldings) and fired up the boil, which by and large went fine. Towards the very end, I started seeing the massive foaming that normally happens when I reduce first runnings to syrup - had I boiled this beer much longer, I would have gotten into that territory. Needless to say, my wort was quite dark.

Darker than a typical stout, in fact.
As opposed to my cooler setup, where I can pretty much guarantee around 75 percent efficiency, I had no real idea as to how my efficiency would work on this stovetop/no sparge setup. As a result, I spitballed 65 percent efficiency on my recipe. I ended up overboiling just a bit (I was about a quart low in final volume), but even allowing for that, I overshot my gravity (1.067 vs a target of 1.063) - for future experiments, I'll probably use the 69 percent that I observed instead.
I added a quart of water back and transferred everything to a sanitized bucket. Silas (age five) and Wynter (age three) demanded to handle the oxygenation duties, so I let them each take turns running about thirty seconds of pure O2 into the batch.

Aeration is serious business!
A portion of my WLP002 starter was then pitched, the lid and airlock were added, and the bucket was placed in my fermentation chamber at 18.3 degrees C/65 degrees F. In a few days, we'll see check and see if we have a reasonable final gravity (the recipe target is 1.017). If so, I'll keg it up, give it a couple of days, and see if it's even remotely drinkable.
I will say - I was surprised at just how roasty the wort smelled. The flavor was... dark, bitter, and not super pleasant. I'll be interested to see how fermentation treats this one. For my purposes, success will be measured by a reasonable final gravity - proving that the sugars were, in fact, already converted - and by a beer that is at least drinkable. I should have results in a couple of weeks!
Share this recipe on reddit
Permalink
Tags for this post: crystal, malt, 90 percent, pushing, limits, homebrew
1 Comments
How did it taste and ferment?
posted by edaisu on 5/03/2017 at 09:26:14 AM
Crystal malts are produced through a unique process; barley is steeped and germinated, then heated in a closed system that does not allow moisture to escape. The malt is held at temperatures where the amylase enzymes are activated, which converts the starches of the malt to simpler sugars - you may recognize this as the exact same reactions that take place during your mash. After this process, the grain is then kilned; the high heat caramelizes some of the sugars in the grain, which is why crystal malts are often referred to as "caramel" malts. The higher the kilning temperature, the darker the crystal malt; differences in kilning temperatures result in different flavor profiles from the caramelization that range from simple sweetness, to caramel, to toffee, to burnt sugar, to raisin and stone fruit. Crystal malts also contribute color - which is often varying in levels of red - to a beer.
While most homebrewing literature allows that crystal malts can be used at up to twenty percent of the grist, I have found that it is far more common for homebrewers to try to limit these malts to the five to ten percent range (or less). If I had a dollar for every time I've read testimonies of brewers who claim that even tiny amounts of the lowest lovibond rated crystal malts make beers just cloyingly sweet and undrinkble... well, I would have a little spending money, at any rate.
As it just so happens, caramel is perhaps my favorite flavor in the world. While the cool kids are ordering hot fudge sundaes, I'm the guy getting caramel on mine. I love caramel candies of all sorts (and please - don't ruin my perfectly good caramel with salt), so it should come as no surprise that I'm also fond of caramel flavor in beer. So much so, in fact, that my brewing buddies widely consider crystal malts to be my "thing".
I have never been shy about pushing beyond the commonly accepted usage thresholds for crystal malts, and even beyond the guidelines suggested by maltsters. One of my house beers is a British brown ale that features about twenty-two percent crystal malt. I have been told many times that surely my beer is undrinkably sweet - cloying and sticky are terms I've heard associated with it. Yet, the beer in question finishes below 1.010, ensuring that it is instead fairly dry. It does indeed have strong caramel flavor, but the beer isn't actually sweet by any stretch of the imagination.
A year ago, one of my brewing buddies latched on to some unclear language for the 2015 BrewUnited Challenge and decided that he should be allowed to brew a 100 percent C60 beer. We clarified the language, but I told him to go ahead and brew it if he wanted to do so, that I wouldn't disqualify him due to his technical loophole. Well, he backed off, but he did brew a 50 percent Special B beer (Special B is a very dark crystal malt, if you were not aware) for fun, and as a joke on me. We all assumed that his beer would be undrinkable, but he reported that while it wasn't really to his taste, it did present as a passable porter if you squinted at it just so.
A few weeks ago, I was mulling over some ideas for some fun beer experiments to try, why my friend's 50 percent Special B beer popped into my head. If he could brew a drinkable beer with 50 percent crystal malt, what was the actual ceiling?
And so, the idea for a new series of posts was born. I'm going to use the super unimaginative tag "Pushing the Limits" for these posts, in which I'll take some aspect of brewing and push it beyond the reasonable, widely accepted limits. If all goes well, I should be able to do about one of these experimental brews per month.
Homework on the subject of crystal malt led to some differing opinions. On the one hand, there is the opinion that I shouldn't even have to actually mash the crystal malt, as enzyme conversion already happened during the process outlined above. Another very common opinion is that despite this, crystal malt cannot self convert, that I would need a normal portion of base grain to ensure conversion of those sugars. My own LHBS owner believes that the very lightest C malts can be mashed successfully without much base malt, but that the darker ones will have no diastatic power at all, and will require base grains for success. Clearly, somebody is wrong somewhere!
My original idea was to go with a grain bill that was "only" 75 percent crystal malt, with the balance made up of Maris Otter. When I floated this silly idea to my brewing buddies on the BrewUnited email list, they mocked me a bit, but also encouraged me to go for broke (Marshall Schott of Brulosophy fame called me all sorts of names for not leaping right to 100% crystal malt). I ended up settling on a compromise of 90 percent crystal malt (split between C60 and extra dark crystal) and ten percent base malt. If this brew turns out successfully, I will bump the crystal malt percentage up - very likely all the way to 100 percent for a future experiment. If it fails, I'll dial things back a bit until I find the point where I do get successful conversion.
According to the most commonly repeated brewing wisdom, this brew should end in failure; the Maris Otter won't have enough leftover diastatic power to convert the sugars from such a high amount of crystal malt. It is also widely believed that my beer is going to finish with an unacceptably high final gravity and some overwhelming sweetness. Some other experiments suggest that the conventional wisdom is wrong, that enzyme conversion really does happen during the production process, that all I need to do is extract those sugars. Experience also tells me that crystal malts may slightly raise final gravity, but that it should be possible to make a beer with a lot of crystal malt that avoid being cloyingly sweet. There is, of course, one way to find out!
Understand that while the muse had in fact grabbed my imagination, there was no way that I was going to risk a full five gallon batch on a silly idea like this. Instead, I decided to brew a tiny, simple batch - I would brew this as a full volume BIAB batch on my stovetop, do no sparge, then dump it into one of my little two gallon fermentation buckets. I'll keg it up when fermentation is complete, and I should end up with about sixteen glasses worth of beer when I'm done. I can pull a few glasses from the keg, and if it's worth keeping, I'll bottle the remainder out of the keg. That way, I can turn it over quickly for another experiment for next month's brewday. Scaling things down like this also made it possible for me to tack this brew onto my normal planned brewday; this month, I was brewing my British brown ale for NHC. This really fit perfectly well, as I was able to bump the size of my starter up a bit and have yeast ready for both batches.
Brewday came, and my wife had left to go eat and shop with her mother and sister. Since she's not a huge fan of brewing smells, I decided that it made more sense to do the experimental (indoor) batch first. I pulled up Bru'n Water, punched in my grain bill... and started to panic.
Even with zero acid additions, my predicted mash pH was a ridiculously low 4.4. I sent a frantic email to the guys on the BrewUnited email list, explaining my dilemma. How could I add alkalinity without chalk or pickling lime? I did have calcium carbonate, which I was sure added alkalinity, but there wasn't a spot on Bru'n water for it. Oh noes!
Matt Chrispen of Accidentalis was kind enough to point out to me (without insult, even) that calcium carbonate is chalk. Ahem. I knew that. At least the guys didn't mock me like I really deserved.
This did lead to an interesting conundrum. Adding enough chalk to raise the pH to an acceptable level meant that my calcium concentration was going to be higher than I had ever heard of. Check this water profile:
- Ca: 257.6
- Mg: 5.2
- Na: 14.9
- SO2-4: 50.1
- Cl: 62.3
To achieve this water profile (the brown malty profile in Bru'n water was my target), I ended up needing nearly nine grams of salts for my paltry 1.75 gallon batch - of which, almost six grams were calcium carbonate alone. This seemed crazy to me.
I added campden to treat the water, then stirred in the salts. The results were... interesting.

That doesn't look super appetizing...
The grains were dumped into my Brew Bag. I was a little worried as my Brew Bag is sized for my big Coleman xTreme cooler, but there was no way I was using my "plain Jane" grain bag with its big, ugly mesh.
Mashing in was pretty simple. I did overshoot my initial strike temperature, but I sat the pot into a sinkful of cool tap water for a couple of minutes, and was soon exactly where I wanted to be.

Target of 148 F? For a "for fun" stovetop batch, I'll take it.
I then put the lid on the pot and wrapped it in towels. Despite all my chalk, my ten minute mash pH check came in low (thanks, water company!), but not so low that I was worried.
For a while, I started to think that this super simple method had a lot of appeal. Then, thirty-five minutes in, I checked my mash temp and found it lower than I wanted - 143 degrees F. So I removed the towels and turned the heat on. In virtually no time, I was at a freaking 160 F. I ran cool water and stuck the pot in, got it back to 148 F, and replaced the towels. Now, I remembered the biggest drawback to this method - the difficulty in maintaining mash temperatures!
When the mash was over, I removed the grain bag (making a bit of a sticky mess on the countertop). At least the Brew Bag did keep grain out of the boil, and was super easy to clean for the next batch of the day. I then added my hops (I did one FWH addition of East Kent Goldings) and fired up the boil, which by and large went fine. Towards the very end, I started seeing the massive foaming that normally happens when I reduce first runnings to syrup - had I boiled this beer much longer, I would have gotten into that territory. Needless to say, my wort was quite dark.

Darker than a typical stout, in fact.
As opposed to my cooler setup, where I can pretty much guarantee around 75 percent efficiency, I had no real idea as to how my efficiency would work on this stovetop/no sparge setup. As a result, I spitballed 65 percent efficiency on my recipe. I ended up overboiling just a bit (I was about a quart low in final volume), but even allowing for that, I overshot my gravity (1.067 vs a target of 1.063) - for future experiments, I'll probably use the 69 percent that I observed instead.
I added a quart of water back and transferred everything to a sanitized bucket. Silas (age five) and Wynter (age three) demanded to handle the oxygenation duties, so I let them each take turns running about thirty seconds of pure O2 into the batch.

Aeration is serious business!
A portion of my WLP002 starter was then pitched, the lid and airlock were added, and the bucket was placed in my fermentation chamber at 18.3 degrees C/65 degrees F. In a few days, we'll see check and see if we have a reasonable final gravity (the recipe target is 1.017). If so, I'll keg it up, give it a couple of days, and see if it's even remotely drinkable.
I will say - I was surprised at just how roasty the wort smelled. The flavor was... dark, bitter, and not super pleasant. I'll be interested to see how fermentation treats this one. For my purposes, success will be measured by a reasonable final gravity - proving that the sugars were, in fact, already converted - and by a beer that is at least drinkable. I should have results in a couple of weeks!
Share this recipe on reddit
| Batch Size (gallons) | 1.75 | ||||||||||||||||
| Efficiency | 65% | ||||||||||||||||
| Recipe type | All Grain | ||||||||||||||||
| Style | 13B. British Brown Ale | ||||||||||||||||
| Original Gravity | 1.063 | ||||||||||||||||
| Final Gravity | 1.017 | ||||||||||||||||
| ABV | 6.04% (basic) / 6.05% (advanced) [what's this?] | ||||||||||||||||
| IBU | 25.8 | ||||||||||||||||
| Color | 64 SRM | ||||||||||||||||
| Boil Time | 60 min | ||||||||||||||||
| Yeast | White Labs WLP002 (English Ale) | ||||||||||||||||
| Fermentables |
| ||||||||||||||||
| Hops |
| ||||||||||||||||
for complete recipe (with details like mash and fermentation temps), click here | |||||||||||||||||
Permalink
Tags for this post: crystal, malt, 90 percent, pushing, limits, homebrew






Please support BrewUnited by using our Amazon affiliate link when doing any shopping there - be it for homebrewing or for your regular shopping!
1 Comments
How did it taste and ferment?
posted by edaisu on 5/03/2017 at 09:26:14 AM